One thing holding back e-waste recycling is the actual recycling process itself. We need cheaper, safer, cleaner, or more effective methods of separating and recovering the valuable elements from electronics before we can make the whole endeavor more attractive and profitable. Some current methods use large amounts of energy to melt components down, but chemistry could provide some tempting alternatives.
A new study led by Yeongran Hong of the Korea Advanced Institute of Science and Technology involves a chemical with an impressive affinity for gold. Subject some circuit boards to an acid treatment to release its materials and this stuff will gather up all the dissolved gold. And after it lets go of that gold, its ready to be used again.
The researchers gold-scrubber is based on an organic compound called a porphyrin. Linked together in a polymer, it possesses lots and lots of little pores that, energetically, want to host a metal atom. Thats the kind of structure chemists look for to help with recycling.
The researchers put their polymer through a number of different tests to work out which metals it worked best on and how much it could capture. Its most effective with a small number of precious metals, most notably gold. In fact, compared to the number of pores in the polymer, they found it was capturing about 10 times as many gold atoms. For other elements like platinum, each pore only hosts one atom (responsible atomic social distancing, shall we say). But gold atoms seemed to make a party at each pore.
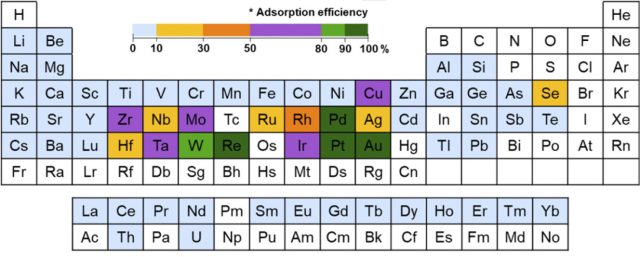
That behavior was verified by measurements and explained by some modeling. The researchers found that the polymer would interact with the gold atom—aided by ultraviolet light—and hand it some electrons, which happens to make it possible for more gold atoms to join in a clump. Sure enough, repeating the test with varying amounts of ultraviolet light had an impact, although capture was still quite high even without it.
Finally, the polymer was put through a pretty authentic test. The researchers took seven circuit boards from a junkyard and put them in an acid bath to leach out the metals. Then they mixed in their polymer, adjusted the solution, and kept it stirring for a couple days. (Although other tests showed that 99 percent of gold can bRead More – Source
[contf] [contfnew] 
arstechnica
[contfnewc] [contfnewc]







