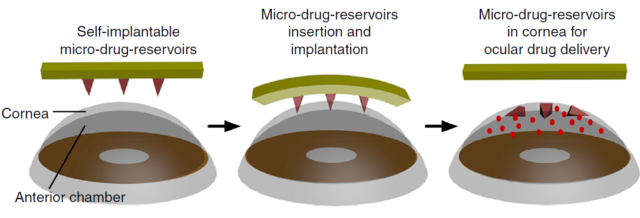
Patients with certain chronic eye diseases, like glaucoma and macular degeneration, typically either use inefficient eyedrops or have to get injections directly into their eyes. A team of researchers wants to replace these with a small patch patients can place on the eye, much like a contact lens. When removed, the patch leaves behind microneedles, which slowly dissolve in the corneal fluid, releasing drugs into the eye as they do. They claim it could make treatments more accessible and less painful for patients, while also providing a more controlled release of the drug.
Replacing injections with a patch
Getting drugs into the eye is a challenge. The most effective solutions tend to be unpleasant and involve actual injections into the eyeball. If doctors deliver the injection in a less horrifying part of the body, it can take a dangerously high initial dose to ensure that enough of the drug actually reaches the eye. Eye drops tend to wash out, and theyre surprisingly inefficient: the eye only absorbs about five percent of the drug most of the time. For some patients, these tradeoffs lead to a choice between needles and blindness.
Some recent ideas have focused on contact lens-like hydrogels that keep drugs on the surface of the eye longer than eye drops to surgically implant tiny drug reservoirs into the eye. Others have involved microneedles: pyramid-shaped needles just a few hundred micrometers long. Microneedles have been increasingly popular as a less-painful way to get drugs through a patients skin without an injection—theyve been used to deliver vaccinations, local anesthetics, and anti-diabetic medications.
More recently, theyve been tested on eyes. But most of those experiments have used stainless steel microneedles coated with medicine or hollow glass ones filled with it. The microneedles get the drug into the eye, but they release it quickly, and the natural circulation of fluid in the eye clears away most of the drug before it can be absorbed; its the same problem that limits injections, eye drops, and hydrogels. A slower, longer release of the drug is usually more effective, especially for chronic, degenerative conditions.
To avoid these limitations, biologist Peng Chen and his colleagues wanted microneedles that would get into the upper layers of the cornea and then dissolve there.
Borrowing materials from the body
They turned to a molecule called hyaluronic acid (HA), which is found naturally in the bodys connective tissue and in the vitreous fluid of the eye. Its transparent and often used to make artificial tears to treat dry eyes, and its a fairly popular choice for dissolving microneedles for transdermal drugs. HA can penetrate skin easily enough, but, against a wet surface like the cornea, it would dissolve and crumple, which isnt helpful if you need a sharp, pyramid-shaped needle to penetrate the outer layers of the cornea.
So Chen and his colleagues combined hyaluronic acid with another molecule called methacrylic anhydride. They used ultraviolet light to trigger reactions that bound the polymer chains together, and the result was a material that could resist dissolving long enough to penetrate the wet surface of the cornea and then slowly release its payload over a few days. The material, called MeHA, formed the molded outer layer of the microneedles. Chen and his colleagues added an inner layer of plain HA, which would make the needles stiffer but release its drug payload faster.
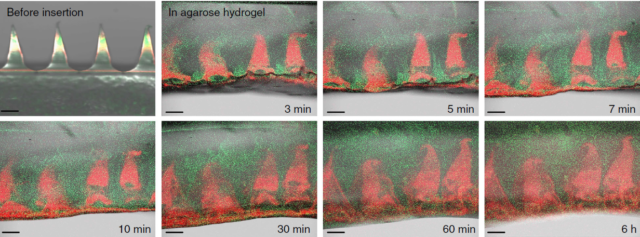
The combination means that the needles can penetrate the outer layers of the cornea, the epithelial and stromal layers, which protect the eye. Once there, corneal fluid seeps into the space between the patch and base of the needles, dissolving the flat base of HA that holds the needles onto the patch. When the patient removes the patch, the microneedles stay behind in the cornea. The HA inner layer dissolves quickly, releasing a burst of the drug within five to 30 minutes, while the outer MeHA layer dissolves more slowly, releasing more of the drug over the next five to six days. This makes sure theres enough of it in the eye to be effective.
Two-layer microneedles could also allow patients to get a quick dose of an anti-inflammatory drug from the fast-dissolving HA inner layer, followed by a more gradual dose of a drug that tones down inflammation more generally. Chen and his colleagues say they could technically also mold a third layer or modify the MeHA compound for slower release.
Fighting corneal disease
An initial experiment in mice looks promising. Chen and his team tested a mouse-sized version of the patch—about 2mm square, with nine 500-micrometer tall microneedles—on mice with a condition called corneal neovascularization. This condition occurs when abnormal blood vessels start growing into the clear tissue of the cornea. An antibody called DC101 helps block a protein thats important in the formation of new blood vessels, preventing their unwanted growth in the cornea.
The dual-layered microneedles, after a few days of dissolving, decreased the area of abnormal blood vessel growth by about 90 percent. A patch with a simpler version of the microneedles, molded with a single layer of HA, decreased the area covered by the blood vessels by a bit less than half. Thats about the same result as injections or earlier experiments with steel microneedles coated with the medicine; eye drops had almost no effect, even at a relatively high dose.
Having microneedles dissolving in your eye sounds pretty unnerving, but Chen and his colleagues say most patients wont notice much. You cant really ask a mouse if something hurts, but researchers who work with lab mice have developed a scale for gauging pain based on their behavior, and none of the mice in the study showed any discomfort. And the microneedles were nearly invisible since HA is largely transparent—almost exactly as transparent as the cornea and vitreous fluid in the eye, in fact.
Promising but a long way to go
The patches and microneedles would have to be scaled up for human eyes, which are much larger and equipped with much thicker corneas than mice have. Chen and his colleagues say human patients would need about a hundred microneedles, each around 800 micrometers long and 400 micrometers wide at the base (we know from previous studies that a pyramid with a 2:1 aspect ratio is ideal for microneedles that need to penetrate tissue).
Corneal neovascularization is a disease that affects the outer layers of the eye. A similar condition, retinal neovascularization, causes weak, poorly formed blood vessels to sprout in the retina, the layers of cells at the very back of the eye. Many of the other diseases requiring injections into the eye, like glaucoma and macular degeneration, also take place in the retina.
“We have not tested our current method for delivering drugs to the retina and posterior chamber of the eye,” Chen told Ars Technica, “although it is possible that the drugs delivered into the cornea can diffuse into the posterior chamber, and it is possible to make our microneedle hard enough to penetrate sclera.” Figuring out whether drugs delivered to the cornea by dissolving microneedles can reach the retina in useful quantities will take more research.
“We are working on optimizing the eye patch (microneedle structures, polymer composition, enhanced stiffness of the needle) for better practical use,” Chen told Ars Technica. Theres much more work to be done in the lab before the patches are ready for human trials.
Nature Communications, 2018. DOI: 10.1038/s41467-018-06981-w (About DOIs).
[contf] [contfnew] 
Ars Technica
[contfnewc] [contfnewc]






